Engineering and Contract Manufacturing
On any given program, your contract manufacturer will make many impactful decisions. You might not want to be involved in every little decision. You also don’t want to worry that they are making incorrect assumptions in a vacuum. Our engineers raise issues, identify options, make suggestions, implement solutions, and share updates.
Design and Development
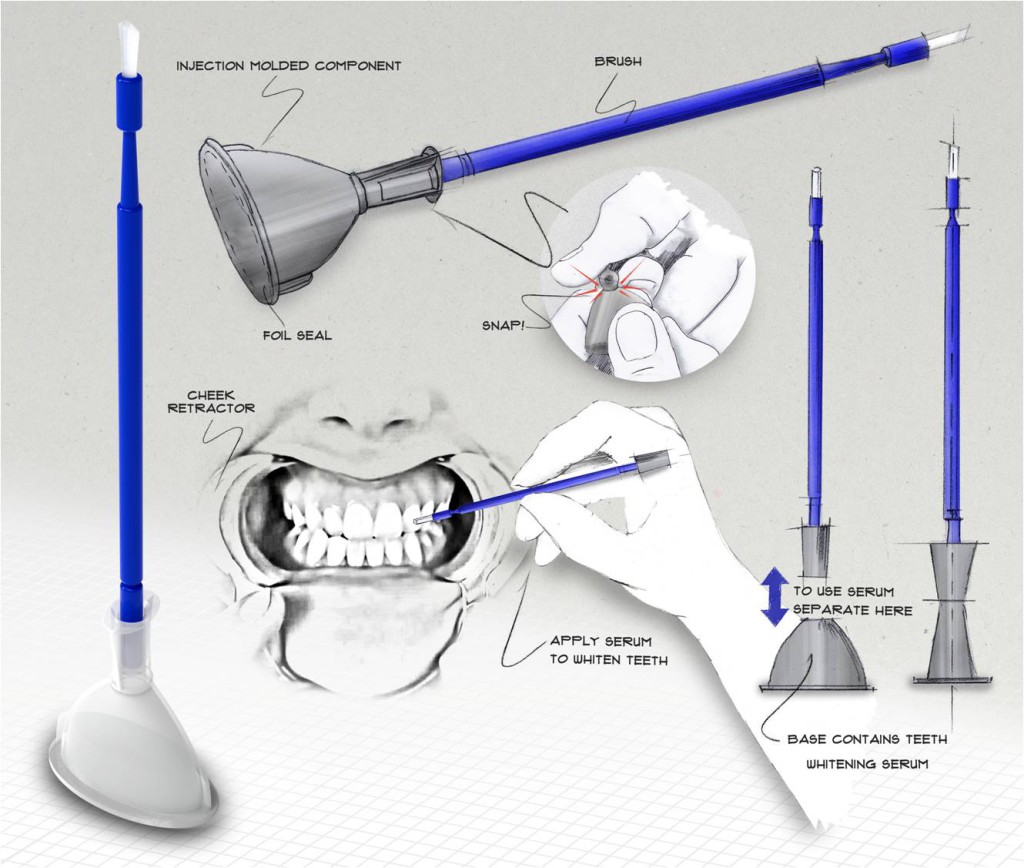
Our customers do not fully understand the injection molding process, so they rely upon the Natech Engineers to help bring their designs to a ready-to-manufacture state. The Natech Engineer integrates the part design, material selection, design for manufacture and assembly (DFMA), and prototyping activities so components are ready to move to the Mold Building Phase.
Explore More


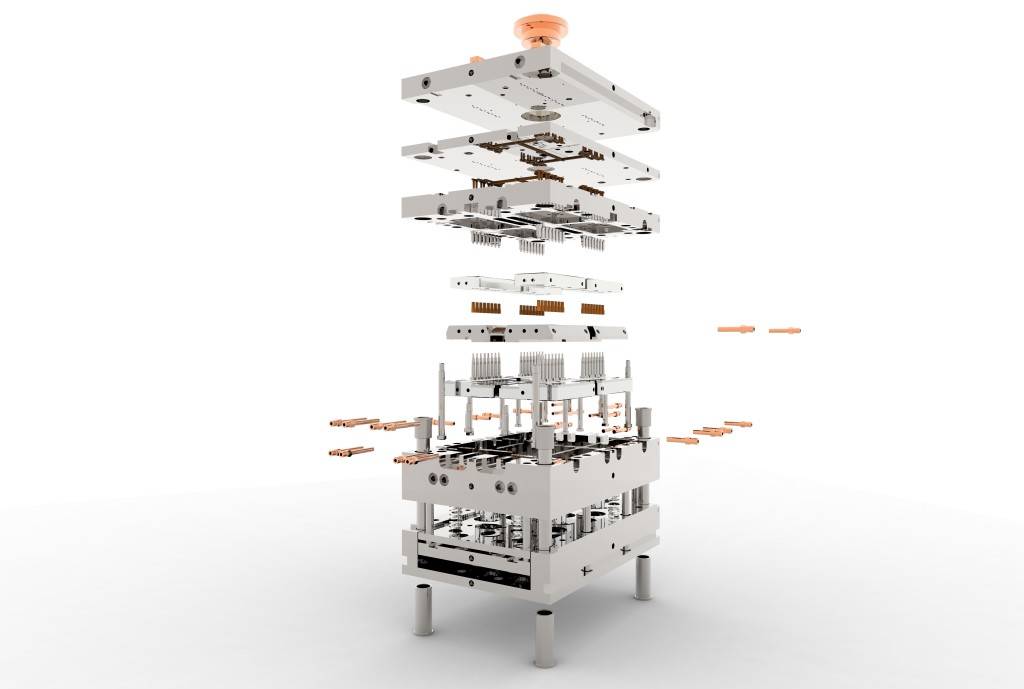
Custom Injection Molds
Too many molds are designed and built either with no optimization or with premature optimization. We believe the mold design defines most of the quality of the mold. A well-designed mold is compact and designed to account for wear, maintenance, and repair over the course of the mold’s life.
Explore More


Mold Qualification
Conventional molding develops a single injection molding process. This approach bears high risk to the quality of the part because a slight variation in the process could produce nonconformities. We use Scientific Molding to establish a full processing window that allows for variation within that process to yield quality at high speed.
Explore More


Custom Injection Molding
Natech Plastics invests in the equipment, systems, and people who keep the company young, vibrant, and on the technological forefront of custom injection molding, assembly, decorating, joining, filling, sealing, and packaging.
Explore More


Contract Manufacturing
Many product manufacturers have suffered through the pains of low-quality assembly, decorating, joining, filling, sealing, and packaging. We believe the key to quality in contract manufacturing is to be the best at respecting and developing our people. We accomplish this through our preeminent skills mastery program where we develop master learners to become master teachers.
Explore More


Design and Development
Our customers do not fully understand the injection molding process, so they rely upon the Natech Engineers to help bring their designs to a ready-to-manufacture state. The Natech Engineer integrates the part design, material selection, design for manufacture and assembly (DFMA), and prototyping activities so components are ready to move to the Mold Building Phase.
Explore More


Custom Injection Molds
Too many molds are designed and built either with no optimization or with premature optimization. We believe the mold design defines most of the quality of the mold. A well-designed mold is compact and designed to account for wear, maintenance, and repair over the course of the mold’s life.
Explore More


Mold Qualification
Conventional molding develops a single injection molding process. This approach bears high risk to the quality of the part because a slight variation in the process could produce nonconformities. We use Scientific Molding to establish a full processing window that allows for variation within that process to yield quality at high speed.
Explore More


Custom Injection Molding
Natech Plastics invests in the equipment, systems, and people who keep the company young, vibrant, and on the technological forefront of custom injection molding, assembly, decorating, joining, filling, sealing, and packaging.
Explore More


Contract Manufacturing
Many product manufacturers have suffered through the pains of low-quality assembly, decorating, joining, filling, sealing, and packaging. We believe the key to quality in contract manufacturing is to be the best at respecting and developing our people. We accomplish this through our preeminent skills mastery program where we develop master learners to become master teachers.
Explore More