The Current and Future State of Medical Device Manufacturing
This is an excerpt from an interview originally published in MPO Magazine on June 2, 2021 by Sam Brusco, Associate Editor
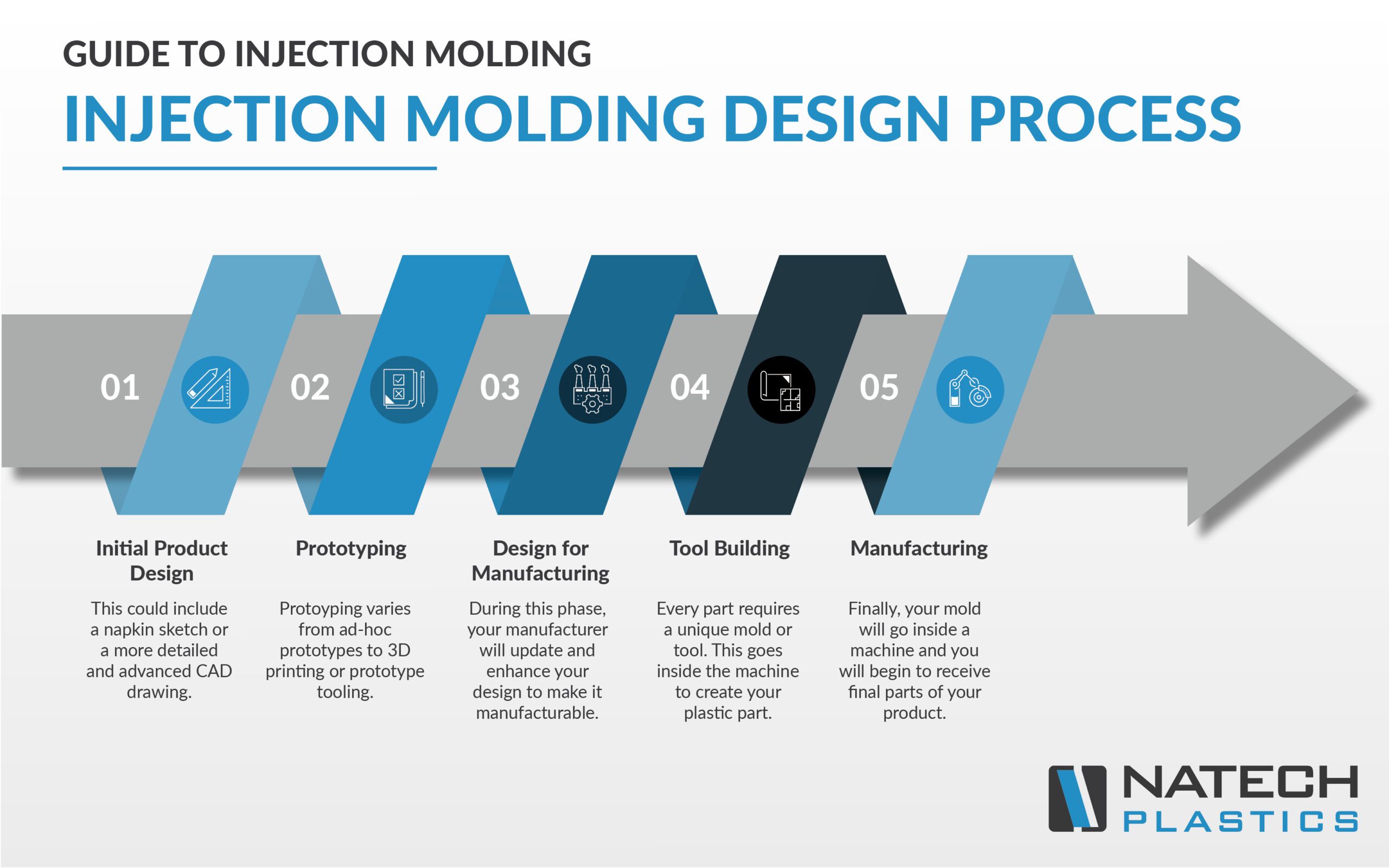
As medical device designs continue to evolve, more molded products and parts are entering the market. Laser-based surgical tools, micro-implants, and bioresorbable technologies are just some of the innovative technologies being molded. Fabricating smaller and more complex medical devices requires extensive experience and high precision from molders.
Molders must also have a thorough knowledge of material behavior, particularly for advanced materials. Medical device makers want to work with molders dedicated to manufacturing medical products or else dedicate a large portion of their business to medical manufacturing.
Rather than focusing on one or two process aspects, many medical molders collaborate with medical device customers. They may offer services including design, prototype development, guidance on design for manufacturing (DFM), material selection, and full production. It’s become practice for molders to successfully manage all elements in the process from design to regulatory support.
Parts are also becoming small enough that sometimes a full production run can be held in one hand. Small parts require a molder strong in 2-shot, multi-component, or electronically integrated parts. Medical device manufacturers constantly design more products needing overmolding or insert molding to combine materials—plastic, metal, rubber, or thin, flexible surface electrodes—to create unique physical properties or specific surfaces or textures.
Sam Brusco: What latest advances in molding technologies/supporting technologies have you invested in, and what are they making possible for medical manufacturing?
Ashley Turrell: First is our integration of co-bots into molding workcells. Utilizing co-bots adds various benefits for our team and clients, including eliminating unnecessary labor and human error, while simultaneously increasing our process’s quality and consistency. This ultimately leads to better quality parts and reduced costs for clients.
We recently invested in filling and sealing stations for diagnostics clients. We can now mold their diagnostic devices, assemble them, and, fill them with liquid, foil seal diagnostics parts, appropriately package and ship to clients, all in-house. This helps shrink what’s usually a complicated supply chain, while also mitigating additional costs associated with contracting these services to vendors. Because this process occurs at one New York facility, we control the complete process and ensure quality standards are met at each manufacturing and assembly stage.
We registered with the FDA and installed a Class 8 cleanroom to assemble and package medical devices. We qualified the process using a design of experiments to characterize a full processing window and developed a training program for operators working with the cleanroom equipment.
Sam Brusco: How do you ensure molding remains competitive with other manufacturing technologies (e.g., CNC hybrid machining, additive manufacturing, etc.)?
Turrell: Molding holds an inherent advantage over many other technologies at higher volumes because it can achieve high quality at high speed for complex parts. We have incorporated Scientific Molding principles into our mold qualification process to ensure it remains capable and stable over the long term.
Brusco: What do OEMs look for when choosing a medical molder? Why do they seek out your company specifically?
Turrell: Typically, they choose molders based on responsiveness, industry-specific certifications, industry experience, manufacturing capabilities, values, and reputation.
Companies seek us out because we specialize in partnering with medical device companies and innovative medical startups. We have an engineering team guiding clients from design through final assembly, all the time paired with one project manager that sees the complete project through.
Our extensive medical device and diagnostic experience comforts clients because they know they’re working with a team that truly understands their needs and can help proactively find and mitigate risks.
Medical companies also appreciate our quality management system. We’re certified ISO 9001, ISO 13485, and FDA-registered. Knowing we meet these rigorous quality standards gives clients peace of mind. Our whiteroom manufacturing space and class 8 cleanroom also intrigue current and potential clients. These spaces are critical for medical device production.
Brusco: Where is molding for medical manufacturing headed? What’s coming in the next few years?
Turrell: Medical manufacturing continues “breaking the rules” for what’s possible. Each new part geometry brings unique challenges. Features get smaller, tolerances grow tighter, and quantities of those components are rising. The level of accuracy and precision we can hit shrinks and tightens, and we must maintain optimal Cpk (process capability index) over longer runs. We’ve made parts with 20-micron features—if those features are off by too many microns, the device won’t work. We anticipate this trend to keep extending.