Quality by Design for Manufacture

A thorough Design for Manufacture and Assembly (DFMA) can make the difference between a project smoothly transitioning into production and getting trapped in development. For the teams who make this transition work, communication is the differentiator. The primary responsibility rests on the Product Development Engineer to understand and balance the needs of the client, the end user, the mold maker, the manufacturer, and the assembler.
After receiving inadequate support for DFMA at another manufacturer, a medical client submitted their design to the Natech engineers for a unique dental application. An injection molded capsule would be filled and sealed along the base, and end users would break off the tip to access the product. Prior to moving to manufacturing, the client’s concerns centered on the breakability and sealing. After the initial review, Natech engineers identified ejection of the part from the mold as a third risk to address during the DFMA process.
Breakpoint
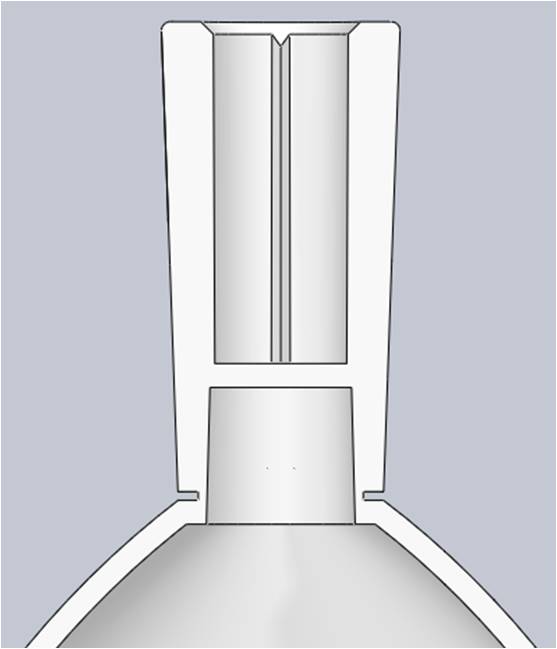
To ensure repeatability, the breakpoint feature would have to have a concentrated area of weakness relative to a surrounding area of strength. To create the area of weakness the Product Development Engineer reduced the wall thickness around a focused diameter along the base of the neck. The thick, uneven walls in the neighboring features posed concerns about bubbles and deflection. These sections were cored out to create support ribs, which improved overall moldability.
With a more optimized breakpoint design, the Product Development Engineer also wanted the flexibility to modify that feature if necessary once molded parts were tested. To accommodate this the mold design would include an interchangeable blade in the R&D mold to more efficiently test different geometries with mold inserts.
Ejection

During high production molding, the parts must properly eject from the mold. This is crucial, because a reliable ejection impacts the cycle time and ultimately the piece price. On a high-cavitation mold, this impact is multiplied.
The Product Development Engineer identified the risk that if the strength of the flange were weaker than the force necessary to push the part off the core of the mold, the ejector pins could deform the flange. As resin cools, a part generally contracts and tends to stick to the core, so the ejector pins will push the part off the core. Performing this ejection motion on a weak feature could result in a degradation of the part quality.
Two ribs were added above the ejection areas of the flange for reinforcement. These were recessed from the edge and followed the main body contour for improved aesthetics.
Sealing
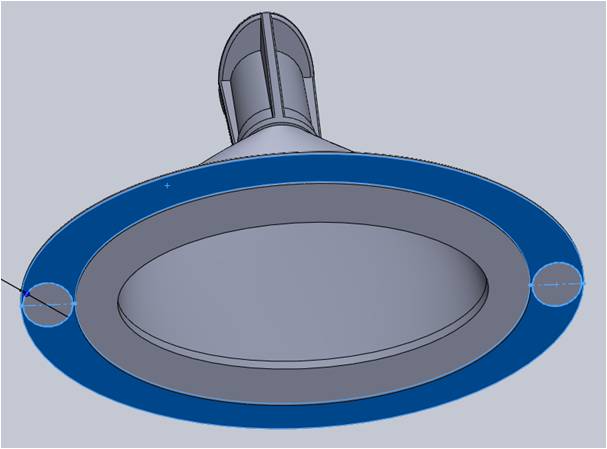
The bottom of the flange along the base served as the surface for both ejecting the part from the mold as well as the sealing surface of the part after filling. The mold’s ejection pins could create small variations in the surface smoothness, which can pose as a risk to adequate sealing.
To address this risk, the Natech engineer increased the flange area to accommodate the two ejector pins. This allowed for a balanced ejection while maintaining smoothness over an adequate surface area for sealing.
The single-cavity sampling and run of 50,000 units showed the risks had been adequately addressed during design. The success of the initial run of 250,000 units on the high-cavitation mold validated the design.
To have compensated for these issues during production, either the mold or the process would have changed. Identifying and handling these risks during the design phase helped avoid these delays and costs later. The Product Development Engineer must manage the quality, economics, and efficiencies for the product over the long term because stakeholders often must deal with or live with the results long after a project is complete.
To learn more contact David Kachoui at dkachoui@natechplastics.com or call (631) 580-3506.
