What to Ask Their Quality Team Before Committing to a Medical Manufacturer
Before you start working with a manufacturer, you might ask them about their overall capabilities and services. Do they have the equipment to manufacture your product? Do they have the appropriate certifications? Do they have the most reasonable price? However, one thing companies often fail to ask about is how manufacturers control product quality.
All too often, we hear clients share that they’ve been burned on quality by previous suppliers. When it comes to receiving great quality products, it is critical to examine how a supplier integrates quality into their systems.
Here, we’ll share 10 questions you should ask their quality team to ensure your expectations are met at every stage of development and manufacture.
1. What are your quality certifications?
If you’re planning on producing medical devices, this is an important question to ask your manufacturer. Certain certifications guarantee that medical manufacturers have all the requirements for standardization, quality assurance and consistency.
ISO 13485:2016 and ISO 9001:2015 certifications ensure your team is implementing and adhering to strict quality management systems and protocols in all processes. These structures ensure your parts conform to specification by maintaining repeatable and traceable processes.
Read: Learn How These Certifications Ensure Continual Improvement
2. When is quality implemented?
Quality should be implemented throughout your product’s lifecycle. A robust quality process approach examines quality starting at the very beginning, in the product design phase all the way through final product manufacturing.
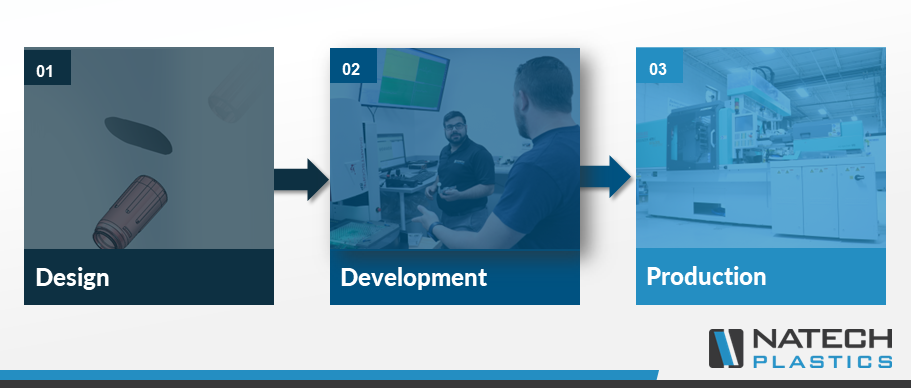
It’s important to identify quality requirements that will impact product functionality early in the project development, so your manufacturer can continually identify and mitigate risks.
3. How is quality implemented during product design?
From Day 1, your manufacturer should aim to eliminate defects and part risks. Incorporating quality at the design for manufacturing phase ensures that parts are not only designed to meet your requirements today, but also designed for repeatable quality and efficient manufacturability.
Download: Design for Manufacturing Checklist
Read: 4 Design Solutions that Improved Manufacturability
4. How is quality tracked during design?
You want your manufacturing partner to have structures and processes in place that track quality from the beginning. Ask about critical-to-quality (CTQ) characteristics, which allow quality teams to track functional features and key performance indicators over the life of your product. This makes sure that any potential non-conformances found during production are identified and fixed.
To identify the method of data collection, your team should identify the means for logging specification requirements, performing functional testing, incorporating AQL sampling, and tracking defect reports.
During the initial design phase, your team should conduct project discovery and collaborate with you to understand your specific product’s goals and requirements.
Read: Why Startups Should Be Thinking About Design for Manufacturing
5. How is quality incorporated in the mold design?
To guarantee your expected volumes, your team should ensure a robust mold design. The robustness of design inputs should maintain product functionality throughout the project’s life.
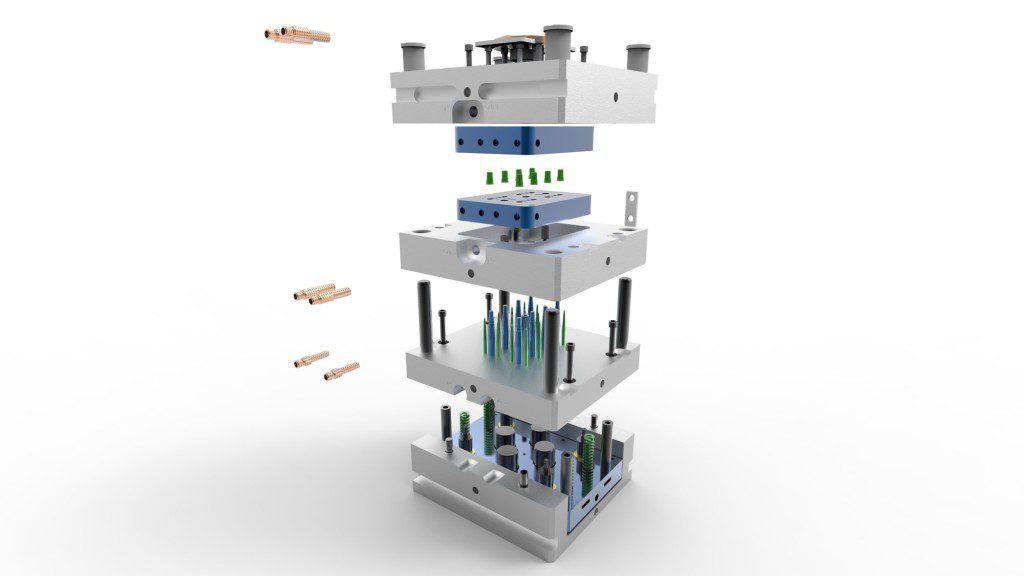
You also want your team to establish material selection and tooling compatibility during design. This will give you the highest level of confidence in your end product’s quality before you start prototyping.
Read: Achieving Quality Mold Design
6. Are quality plans set in stone or can they be customized?
It’s best to find a manufacturer that customizes quality control plans to fit your project’s requirements. During process development, top quality teams can qualify equipment and processes, create documentation, establish product specific QC plans, and implement job-specific work instructions before beginning manufacturing.
Ask your manufacturing partner if they ensure documentation of:
1) Assembly instructions
2) Packaging requirements
3) Methods to verify product
Does your team develop work instructions for project specific equipment or maintenance activities? For more complex applications, do they provide robust job-specific training to operators and personnel who handle the product? A team should be qualified to make informed decisions about how the process is performed. This way, you can have confidence in your final parts.
7. What do your QC plans consist of?
QC plans typically have a minimum of three sections, including the first in-audit inspection, in-process inspections, and final inspection. These plans tailor a specific set of tasks that alert team members of critical issues that could impact product conformity.
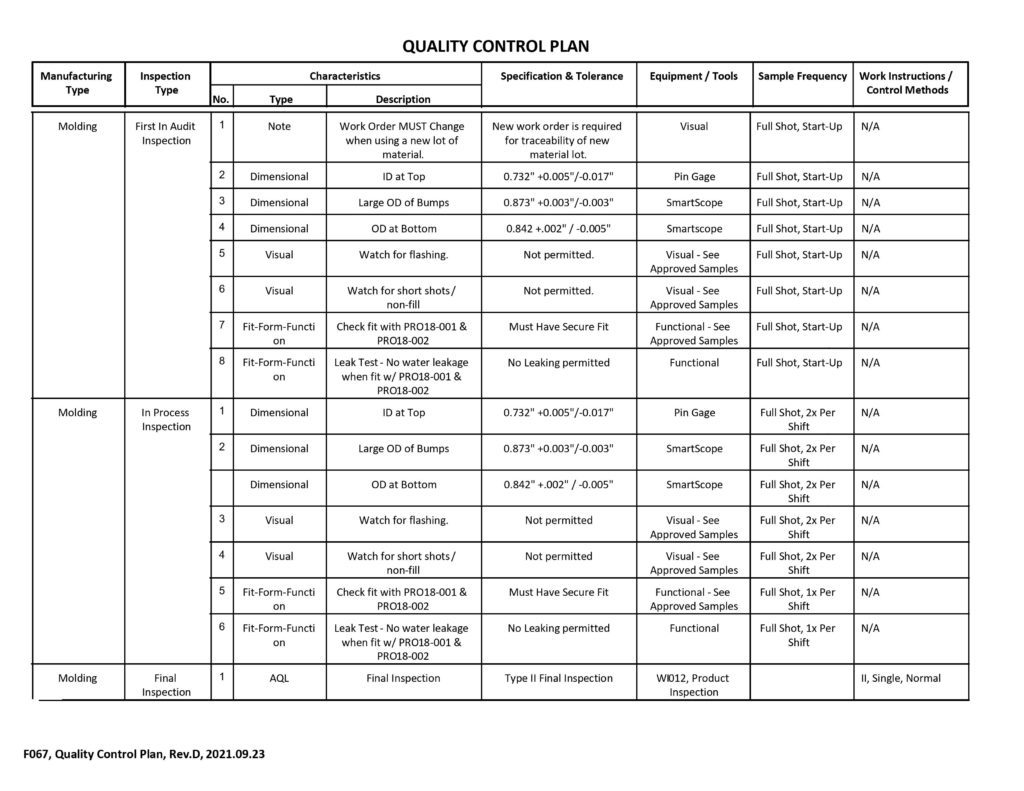
Ask if their team creates custom fixturing or gage stations for parts intended to test for form-fit-function or other specific quality and manufacturing requirements. This can include assembly fixtures, functional test jigs, and verification procedures.
8. How is quality incorporated into production?
To ensure machinery outputs are consistent with quality requirements, you want their operations team to verify equipment and process parameters. During production, the quality team should verify the product by inspecting, while following established QC plan criteria.
Ideally, their team should adhere to documented processes to control any nonconforming products. If a nonconformity is discovered during inspection, you want a team that will immediately take action to identify the source of error, separate the affected product and apply corrective or preventative action to the process.
Read: How to Achieve Quality Through Process Validation
9. What kind of inspection tools do you have?
Experienced teams will have a wide range of equipment. These tools can range from high-level, automated tools, like Smartscopes and vision systems, to specialized tools, like leak chambers and force gauges, to standard inspection tools. These tools are used for in-process inspections for process control certainty.
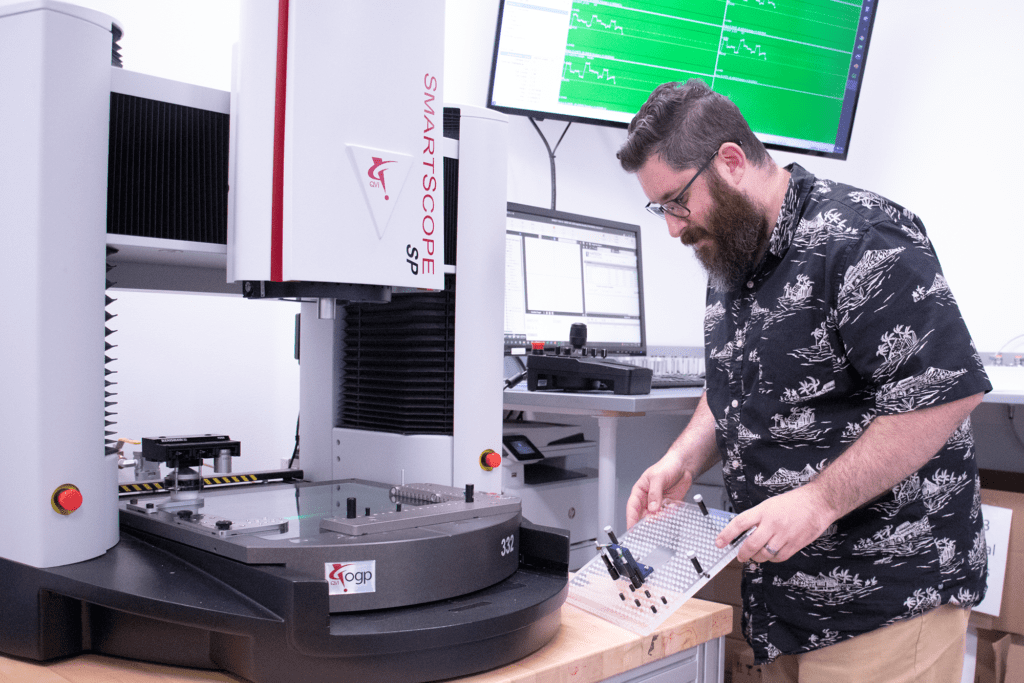
Read: 4 Tools that Ensure Quality in Complex Parts
10. What is your in-process inspection like?
Make sure your product is cared for after it’s produced. You want a trained, qualified team to regularly perform inspections throughout your production run.
Ask how often their team checks products for visual, dimensional, and functional specifications requirements. Rigorous inspections at predetermined intervals help to quickly discover aesthetic or dimensional defects, such as sink marks, scratching, warpage, splay, or tool-related issues, such as occluded thru-holes, flash, high gate, or poor geometry formation.

Additional inspections may take place at a predetermined AQL level. An effective final inspection plan for packaged products ensures that the product is safe from damage during transit or handling.
Natech collaborates with you to create an effective plan with any combination of inspections to best fit your project needs. At the end of your project’s journey, you should have confidence in the quality of the product you receive from Natech.



