High-performance Applications
Natech delivers high-performance, critical-tolerance applications with plastic injection molded components that require a high engineering touch. An engineering strength in Design for Manufacture (DFM) and Design for Assembly (DFA) provides the basis for time and cost savings. Clients will frequently come to Natech, having already built prototypes or molded components that do not work because the DFM/DFA step was skipped. The same clients sometimes want us to skip the DFM/DFA process. One success after another has illustrated the value of allocating upfront resources to design for manufacturability. The research-minded engineers at Natech document their discoveries to add to the organization’s learning. We share this knowledge with our clients.
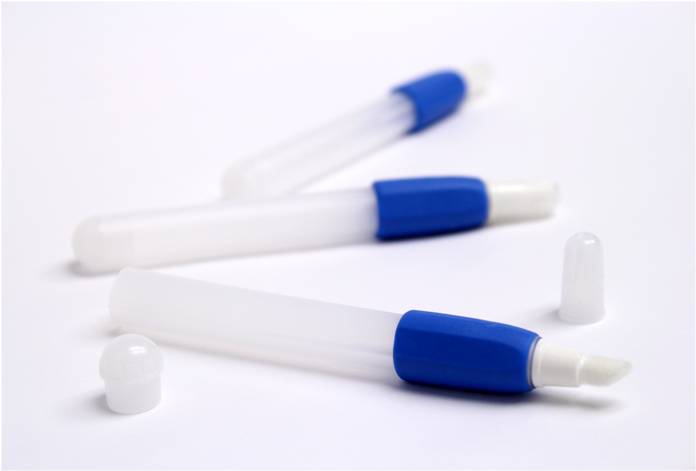
Liquid Seal for Pharmaceutical Package
A pharmaceutical client required a drug delivery package for a combustible material. The liquid was filled and sealed in a glass ampoule to be broken via pinch grip. For the package design, the client recruited another company who delivered a concept with over eleven separate components. The Natech engineers performed the Design for Manufacture and Assembly to reduce the design down to only two components. An overmold was added along with a protective cap.
Explore More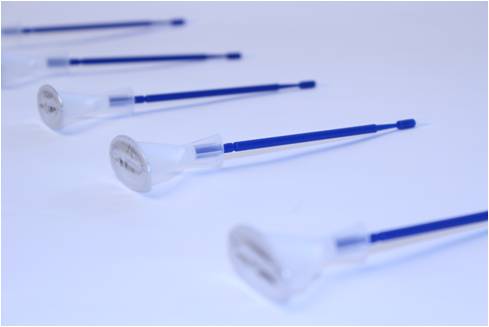


Single-use Dental Delivery Package
A dental applicator required a novel delivery package for a high-end single-use / unit-dose delivery. The liquid product was filled and sealed. The consumer would snap off the tip to access the product just prior to use. Prior to engaging the Natech Engineers, the client was unable to achieve the right breakage for the snap feature.
Explore More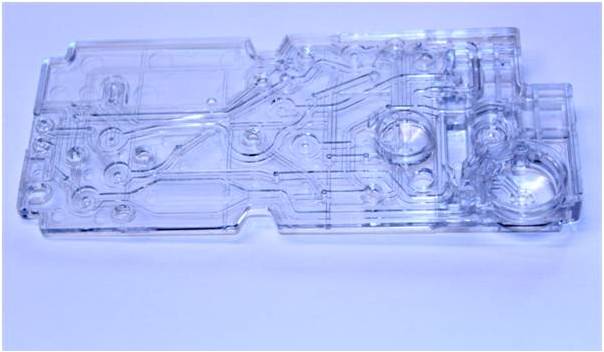


Lab on a Chip Device
A microfluidics client needed a custom injection molded card with tight-tolerance features throughout the component. The flatness requirement and thin-walled features posed challenges to the previous molders of the part. With a thorough design for manufacture phase supported by Mold Flow analysis, Natech was able to achieve a stable process to produce non-DNA, non-RNA parts for the client.
Explore More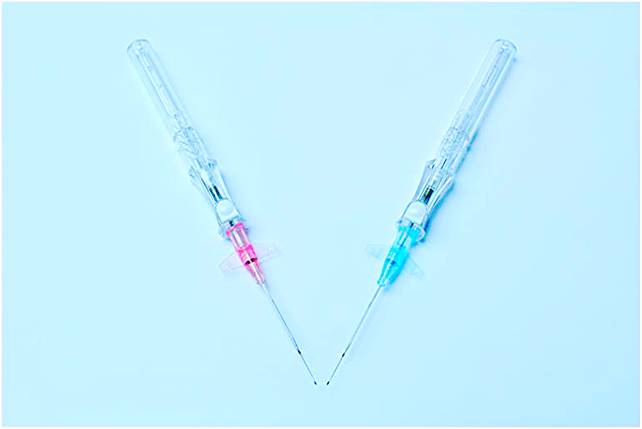


DFM for Medical Micromolding
A client required medical molding for a catheterization device with nine micro molded components. One of the components required a secondary operation to form a feature, which had been deemed unmoldable until the Natech engineers completed the design for manufacturability, yielding great savings for the client.
Explore More


DFM for Consumer Electronics
A client had designed ribbing features to an electronics housing to ensure the necessary strength and flatness properties would be achieved. The client was uncertain about the level of ribbing necessary and erred on the side of excessive ribbing. The Natech Engineers performed a Design for manufacturability analysis to reveal that the excessive ribbing posed major ejection risks. The Natech Engineers reduced the number of ribs as well as other features, which reduced both risks as well as costs due to the amount of resin consumed by the part.
Explore More


Liquid Seal for Pharmaceutical Package
A pharmaceutical client required a drug delivery package for a combustible material. The liquid was filled and sealed in a glass ampoule to be broken via pinch grip. For the package design, the client recruited another company who delivered a concept with over eleven separate components. The Natech engineers performed the Design for Manufacture and Assembly to reduce the design down to only two components. An overmold was added along with a protective cap.
Explore More


Single-use Dental Delivery Package
A dental applicator required a novel delivery package for a high-end single-use / unit-dose delivery. The liquid product was filled and sealed. The consumer would snap off the tip to access the product just prior to use. Prior to engaging the Natech Engineers, the client was unable to achieve the right breakage for the snap feature.
Explore More


Lab on a Chip Device
A microfluidics client needed a custom injection molded card with tight-tolerance features throughout the component. The flatness requirement and thin-walled features posed challenges to the previous molders of the part. With a thorough design for manufacture phase supported by Mold Flow analysis, Natech was able to achieve a stable process to produce non-DNA, non-RNA parts for the client.
Explore More


DFM for Medical Micromolding
A client required medical molding for a catheterization device with nine micro molded components. One of the components required a secondary operation to form a feature, which had been deemed unmoldable until the Natech engineers completed the design for manufacturability, yielding great savings for the client.
Explore More


DFM for Consumer Electronics
A client had designed ribbing features to an electronics housing to ensure the necessary strength and flatness properties would be achieved. The client was uncertain about the level of ribbing necessary and erred on the side of excessive ribbing. The Natech Engineers performed a Design for manufacturability analysis to reveal that the excessive ribbing posed major ejection risks. The Natech Engineers reduced the number of ribs as well as other features, which reduced both risks as well as costs due to the amount of resin consumed by the part.
Explore More